Multiple Choice
Which of the following structures is the major contributor to the resonance hybrid of the phenoxide anion?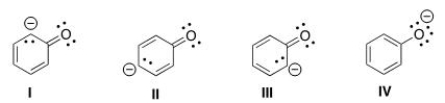
A) I
B) II
C) III
D) IV
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: What is the overall charge of the
Q15: What two groups make up the carboxylic
Q20: Rank the following compounds in order of
Q20: Why is the C-O single bond of
Q21: Which of the following compounds is least
Q23: Rank the following compounds in order of
Q24: What is the correct IUPAC name of
Q28: Which of the following is the correct
Q29: <sub> </sub>What would happen if a mixture
Q45: Why is pure acetic acid often called