Multiple Choice
Determine the geometry around the indicated atom in each species. 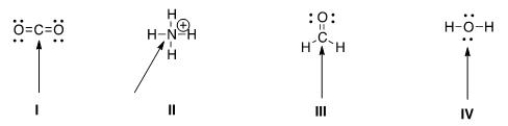
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Q5: What is the ground-state electronic configuration of
Q7: In which of the following ions does
Q8: Which of the following covalent bonds has
Q10: Which of the following molecules has nonpolar
Q14: How many constitutional isomers are there for
Q21: Which of the following statements about resonance
Q34: What is the approximate C-C-C bond angle
Q40: Which of the following statements about bonding
Q61: What is the ground-state electronic configuration of
Q67: Which of the following molecules has polar