Multiple Choice
What is the hybridization for each of the indicated atoms in the following compound?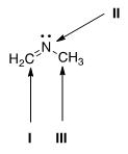
A) I = sp2; II = sp2; III = sp2.
B) I = sp2; II = sp3; III = sp3.
C) I = sp; II = sp2; III = sp3.
D) I = sp2; II = sp2; III = sp3.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which atomic orbitals overlap to form the
Q28: What is the formal charge of carbon
Q31: How many constitutional isomers are there for
Q38: What is the order of decreasing bond
Q39: Which compound contains the most polar bond?<br><img
Q40: Follow the curved arrows to draw the
Q45: Which of the following is the appropriate
Q46: What is the condensed formula of the
Q47: Which of the following Lewis structures is
Q58: What is the approximate bond angle for