Multiple Choice
Identify the particle emitted by the nucleus that undergoes the transformation shown in the figure. 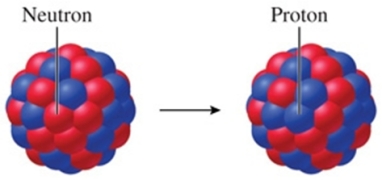
A) an alpha particle
B) a beta particle (electron)
C) a positron
D) a gamma ray
E) a neutron
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: In order for a chain reaction to
Q44: Which of the following statements regarding nuclear
Q64: What product is formed when <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7010/.jpg"
Q65: Why is radon gas dangerous?<br>A)It reacts chemically
Q71: Carbon-11 radioactively decays by positron emission with
Q71: Select the most stable isotope from the
Q72: Fission involves the one, and the release
Q73: Potassium-40 decays by beta emission to form
Q87: The critical mass is the amount of
Q92: The half-life of iodine-131 is 8.1 days.