Multiple Choice
Consider a voltaic cell that corresponds to the following reaction: Cu(s) + 2Ag+(aq) Cu2+(aq) + Ag(s) If this reaction takes place in the electrochemical cell shown in the figure, which of the following statements is incorrect? 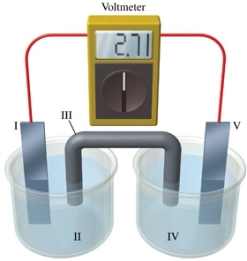
A) I is the copper electrode, which is the anode.
B) II is the Ag+ solution.
C) III is the salt bridge.
D) V contains the substance that is being reduced.
E) V is the silver electrode, which is the cathode.
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Examine the following reaction: 5FeCl<sub>2</sub>(aq) +
Q84: Consider the reaction: Zn(s) + H<sub>2</sub>SO<sub>4</sub>(aq)
Q85: When a piece of copper wire is
Q86: Consider the reaction: H<sub>2</sub>O(l) + 3SO<sub>3</sub><sup>2</sup><sup>-</sup>(aq)
Q87: Given the following information about the
Q89: A lead-acid battery that is used
Q90: Given the following information about the
Q91: Consider a voltaic cell that corresponds
Q93: In which of the following does chlorine
Q99: Examine the following reaction: 5FeCl<sub>2</sub>(aq)+ KMnO<sub>4</sub>(aq)+ 8HCl(aq)-