Multiple Choice
The figure shows the electrolysis of molten AlCl3.What reaction occurs at the cathode of this cell? 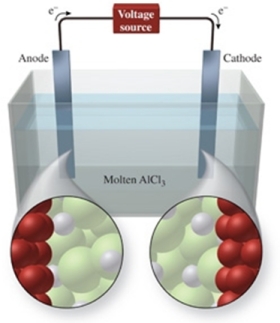
A) AlCl3(l) AlCl2(l)
B) Cl-(l) + e- Cl2-
C) Al3+(l) + 3e- Al(l)
D) 2Cl-(l) + 2e- Cl2(g)
E) 2Cl-(l) Cl2(g) + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Q72: The reaction that occurs in an
Q74: A lead-acid battery that is used
Q75: A mercury button battery that is
Q76: Consider the reaction: Sn<sup>2+</sup>(aq) + 2Fe<sup>3+</sup>(aq)
Q78: Consider the reaction: CrO<sub>4</sub><sup>2</sup><sup>-</sup>(aq) + HSO<sub>3</sub>-(aq)
Q79: The following reaction occurs in acid
Q80: Consider the following reaction: 2Fe<sup>3+</sup>(aq) +
Q81: Consider a voltaic cell that corresponds
Q82: Given the following information about the
Q83: The salt bridge in an electrochemical cell