Multiple Choice
The figure shows the electrolysis of molten AlCl3.What reaction occurs at the anode of this cell? 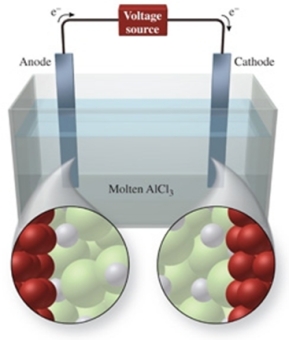
A) AlCl3(l) AlCl2(l)
B) Cl-(l) + e- Cl2-
C) Al3+(l) + 3e- Al(l)
D) 2Cl-(l) + 2e- Cl2(g)
E) 2Cl-(l) Cl2(g) + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Q38: In a voltaic cell, the electron flow
Q66: Which one of the following reactions
Q67: Consider the reaction: Ba(NO<sub>3</sub>)<sub>2</sub>(aq) + Na<sub>2</sub>SO<sub>4</sub>(aq)
Q68: Consider the reaction: H<sub>2</sub>O(l) + 3SO<sub>3</sub><sup>2</sup><sup>-</sup>(aq)
Q72: The reaction that occurs in an
Q74: A lead-acid battery that is used
Q75: A mercury button battery that is
Q76: Consider the reaction: Sn<sup>2+</sup>(aq) + 2Fe<sup>3+</sup>(aq)
Q83: The salt bridge in an electrochemical cell
Q106: When a strip of zinc metal is