Multiple Choice
The H2PO4- ion is amphoteric.What are the conjugate acid and conjugate base of H2PO4-? 
A) 
B) 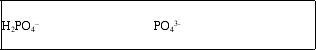
C) 
D) 
E) 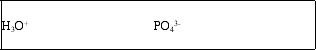
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Select the strong acid from the following
Q94: Given an H<sub>3</sub>O<sup>+</sup> concentration of 1.0 x
Q95: Select the pair that consists of an
Q96: If the pH of a certain soft
Q97: Which of the following is an amphoteric
Q98: Select the two Brønsted-Lowry acids in the
Q100: If the pH of a blood sample
Q101: When the following reaction goes in the
Q103: Select the two Brønsted-Lowry bases in the
Q104: Select the solution below that is the