Multiple Choice
The image shows a molecular-level representation of part of a solution after ammonia, NH3, is dissolved in water.(Besides water, there are 2 NH4+ ions, 6 NH3 molecules, and 2 OH- ions present in the solution.) .Which of the following best describes NH3? 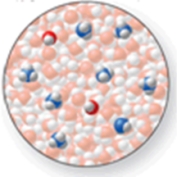
A) strong acid
B) strong base
C) weak acid
D) weak base
E) amphoteric
Correct Answer:

Verified
Correct Answer:
Verified
Q17: If you take an antacid tablet, the
Q32: Which of the following acids is not
Q119: Select the two Brønsted-Lowry bases in the
Q120: List the species present in order of
Q121: If NaH<sub>2</sub>PO<sub>4</sub> is added to water, what
Q122: Select the solution below that is the
Q123: When the following reaction goes in the
Q125: Calculate the pH of a solution that
Q126: Which of the following equations represents the
Q127: Which of the following will not change