Multiple Choice
The image shows a molecular-level representation of part of a solution after HF is dissolved in water.(Besides water, there are 6 HF molecules, 1 H3O+ ion, and 1 F- ion present in the solution.) Which of the following best describes HF? 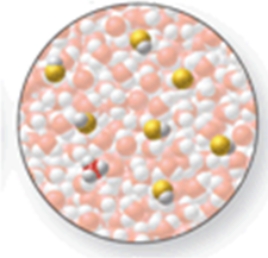
A) strong acid
B) strong base
C) weak acid
D) weak base
E) amphoteric
Correct Answer:

Verified
Correct Answer:
Verified
Q60: Calculate the H<sub>3</sub>O<sup>+</sup> concentration in 0.10 M
Q61: Which of the following ions is amphoteric?<br>A)Cl<sup>-</sup><sup>
Q62: Select the pair that consists of a
Q63: The conjugate base of H<sub>2</sub>PO<sub>4</sub><sup>-</sup> is:<br>A)HPO<sub>4</sub><sup>2-</sup><sup> </sup><br>B)H<sub>2</sub>PO<sub>3</sub><sup>-</sup><sup>
Q64: Rank the following 0.100 M solutions in
Q67: Which of the following statements regarding acids
Q69: When carbonate ion, CO<sub>3</sub><sup>2−</sup>, reacts with water,
Q69: Which of the following is the strongest
Q70: Which of the following ionizes to the
Q92: When acetate ion, CH<sub>3</sub>CO<sub>2</sub><sup>−</sup>, reacts with water,