Multiple Choice
What phase change is occurring in the figure, and is it endothermic or exothermic? 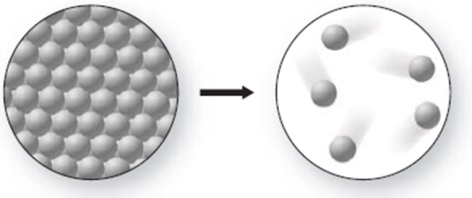
A) melting; exothermic
B) freezing; endothermic
C) freezing; exothermic
D) melting; endothermic
E) sublimation; endothermic
Correct Answer:

Verified
Correct Answer:
Verified
Q34: One would expect that since CO is
Q73: Which of the following statements regarding the
Q82: Which of the following has the highest
Q83: Calculate the amount of heat energy,
Q84: Which of the following statements regarding the
Q88: Calculate the amount of heat energy,
Q89: The molecular-level diagram shows the process of
Q90: Which substance has the highest melting point?<br>A)Mg<br>B)C<sub>5</sub>H<sub>12</sub><sub>
Q101: Which of the following statements is correct?<br>A)A
Q103: If there were no intermolecular forces, most