Multiple Choice
When the mixture of molecules shown in the molecular-level image undergoes complete reaction, all of these molecules are converted to products.Which of the following reactions could this represent? 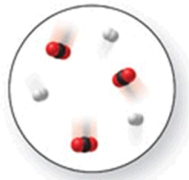
A) N2 + O2 2NO
B) N2 + 2Cl2 N2Cl4
C) N2 + 2O2 2NO2
D) N2 + 3H2 2NH3
E) N2 + 3O2 2NO3
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Which of the following processes is exothermic?<br>A)ice
Q99: The q value for the
Q100: Which of the following is the
Q102: Given the balanced equation 4NH<sub>3</sub>(g) +
Q103: What is the heat change when
Q105: Ammonia is usually made by the
Q106: Small amounts of oxygen gas can
Q107: Given that 4NH<sub>3</sub>(g) + 5O<sub>2</sub>(g)
Q108: Consider the following reaction: 3NO<sub>2</sub>(g) +
Q109: The following reaction releases 2800 kJ