Multiple Choice
Consider the reaction N2(g) + 2O2(g) 2NO2(g) .The molecular image represents a mixture of N2(g) and O2(g) just before reaction occurs.What is the limiting reactant, and how much of the excess reactant remains after the reaction is complete? The image contains 3 N2 molecules and 9 O2 molecules. 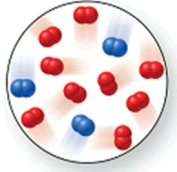
A) N2(g) , 6 O2(g)
B) O2(g) , 1 N2(g)
C) N2(g) , 3 O2(g)
D) O2(g) , 2 N2(g)
E) N2(g) , 7 O2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q35: A calorie used by nutritionists, 1 Calorie,
Q39: When carbon dioxide is formed from its
Q69: When fats or other foods are burned
Q90: A 5.00 g sample of a
Q91: Aluminum metal reacts with sulfuric acid
Q93: Which of the following is the
Q97: Which of the following processes is endothermic?<br>A)burning
Q99: The q value for the
Q100: An energy input of 227 kJ is
Q130: A 43 g serving of a chocolate