Multiple Choice
Consider the energy diagram for the multistep reaction shown here.Which statement about this diagram is not true? 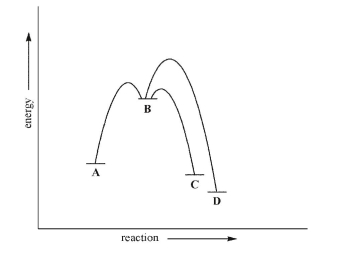
A) C is the kinetic product.
B) D is the thermodynamic product.
C) Given sufficient energy, C and D can equilibrate.
D) Formation of D is under thermodynamic control.
E) Formation of C is under thermodynamic control.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: What is the major S<sub>N</sub>1 product of
Q23: Draw the structures of all possible El
Q24: State the Hammond postulate.
Q25: Draw the major El product of the
Q26: What will happen to the rate of
Q28: What is the major E1 product of
Q29: Draw a mechanism to account for the
Q30: Draw the substitution product that would result
Q31: In the presence of aqueous sulfuric acid,
Q32: Propose a mechanism to account for the