Multiple Choice
Which of the following statements about treatment of an alkyne with sodium metal in ammonia is false? 
A) The product of the reaction is a trans alkene.
B) A radical anion is protonated by solvent.
C) This reaction provides the opposite stereochemistry to that of treating an alkyne with H2 in the presence of Lindlar's catalyst.
D) Sodium amide forms as the reaction proceeds.
E) A single electron is donated by sodium to a 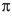 orbital in the alkyne.
orbital in the alkyne.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Draw the product of the following transformation:
Q42: Consider alkenes A and B
Q43: Provide two different precursors and the reagents
Q44: Provide the missing reagents and structures.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q45: The molecule shown was the exclusive product
Q47: Which product will be observed?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Which
Q48: which product will be observed?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="which
Q49: List three ways to accomplish the transformation
Q50: Which of the following is not a
Q51: Explain the difference between a heterogeneous catalyst