Essay
The following transformation was carried out using one enantiomer of diethyl tartrate and the other reagents of the Sharpless asymmetric epoxidation reaction.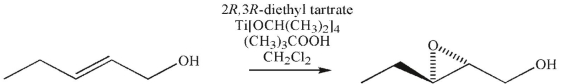
Predict the product that would result using the other enantiomer of diethyl tartrate.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Devise a multistep synthesis of the target
Q21: Which of these products would result from
Q22: Draw the structure of the enol intermediate
Q23: Which of the following structures is an
Q24: Predict the product of the following transformation.
Q26: Devise a multistep synthesis of the target
Q27: Draw a mechanism for the transformation shown.Include
Q28: Which of the following conditions will result
Q29: Show how you would make each of
Q30: How would you make this compound in