Multiple Choice
In the rearrangement of 2-butyne to 1-butyne, what causes the reaction to go to completion?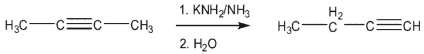
A) the increased stability of the terminal triple bond in the product
B) the high pKa of ammonia versus the methyl group in 2-butyne
C) the decreased activation energy for conversion of the allene intermediate to 1 -butyne
D) the much lower pKa of the acetylenic proton in the product versus the other species
E) the increased stability of the 1,2 -butadiene intermediate versus 2 -butyne
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Draw the structures of the diene and
Q10: Which of these structures is a conjugated
Q11: Which of these compounds can exist as
Q12: Which of these structures is isoprene?<br>A) <img
Q13: Devise a multistep synthesis for the following
Q15: Which of the following molecules could not
Q16: Convert the structure shown here to a
Q17: Draw the exo product of the Diels-Alder
Q18: Which of these alkynes will not isomerize
Q19: Which of the following dienes could react