Multiple Choice
When 1,3 -butadiene reacts with chlorine, a 50/50 mixture of 3,4 -dichloro-1-butene and (E) -1,4-dichloro-2-butene is generated. When the same diene is reacted with bromine, the 3,4 -dibromo-1-butene is the predominant product.. What could explain the difference in regioselectivity?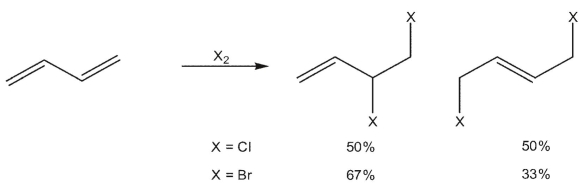
A) The more stable intermediate bromonium ion favors 1,2 -addition.
B) The more reactive bromide anion favors the kinetic product.
C) The more reactive chloride anion favors the thermodynamic product.
D) The more stable intermediate chloronium ion favors the 1,4 -addition product.
E) The electronegative bromine destabilizes the positive charge on the β-carbon.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: Identify the isoprene units in this structure.
Q42: Draw the product of 1,4 addition of
Q43: Draw the products of the addition reaction
Q44: Devise a multistep synthesis for the following
Q45: Which of these orbitals is the HOMO
Q47: Draw a mechanism for the transformation shown
Q48: Which of the following molecules contains the
Q49: Draw the product of 1,2 addition of
Q50: The molecule shown here can undergo an
Q51: Draw the structure of the product formed