Essay
Design a multistep synthesis to produce o-bromoaniline as the sole product using aniline as the
starting material (i.e., you must avoid generating over-brominated products and p-bromoaniline).
You may use any organic or inorganic reagents.Show the reagents needed for each step and the
product of each step. 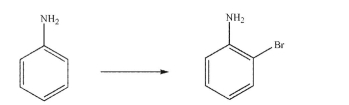
Correct Answer:

Verified
Correct Answer:
Verified
Q22: What is the electrophile in the reaction
Q23: Explain why benzene is inert to treatment
Q24: Draw a mechanism for the transformation shown
Q25: Bromination of aniline results in 2,4,6
Q26: Draw the structure of the electrophile in
Q28: Which of the following statements is true?<br>A)All
Q29: Which of the following compounds is activated
Q30: What is the product of the following
Q31: Predict the product of the following reaction
Q32: A chemist attempted the following Friedel-Crafts alkylation