Essay
For the following reaction: 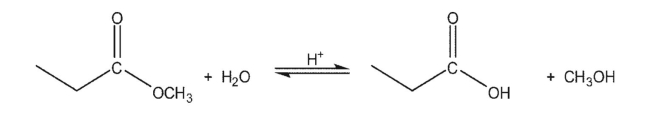 How would each of the following affect the position of the equilibrium?
How would each of the following affect the position of the equilibrium?
A)Running the reaction in methanol as a solvent
B)Running the reaction in a still at a temperature of 85-90 °C
C)Running the reaction in the presence of anhydrous sodium sulfate at room temperature
Correct Answer:

Verified
a.The excess methanol would shift the eq...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q58: What is the product of the following
Q59: Predict the product of the following reaction
Q60: Draw the structure of methyl 3,3-dimethylbutanoate.
Q61: Which of the following statements about amide
Q62: Which of these compounds would have the
Q63: Which of these carbonyl compounds has the
Q64: Which of the following compounds will not
Q66: Match the compound shown to the correct
Q67: Draw a mechanism for the following transformation.Include
Q68: Predict the product of the following reaction