Multiple Choice
The volume V (in liters) of a certain mass of gas is related to its pressure P (in millimeters of mercury) and its temperature T (in degrees Kelvin) by the law 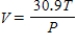
Compute 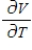 and
and 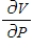 when T = 260 and P = 700.
when T = 260 and P = 700.
A) 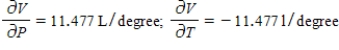 The volume increases by 0.044 L when the temperature decreases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature decreases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
B) 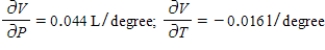 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume decreases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
C) 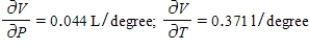 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
D) 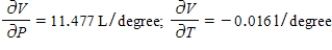 The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
The volume increases by 0.044 L when the temperature increases by 1 degree (beyond 260 K) and the pressure is fixed at 700 mm of mercury. The volume increases by 0.016 L when the pressure increases by 1 mm of mercury (beyond 700 mm) and the temperature is fixed at 260 K.
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Use a double integral to find the
Q40: Maximize the function<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8255/.jpg" alt="Maximize the
Q41: Find the first partial derivatives of the
Q42: Find the approximate change in z when
Q43: Minimize the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8255/.jpg" alt="Minimize
Q45: Find the second-order partial derivatives of the
Q46: Use a double integral to find the
Q47: Use a double integral to find the
Q48: Find the volume of the solid bounded
Q49: Find the critical point(s) of the function.