Multiple Choice
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 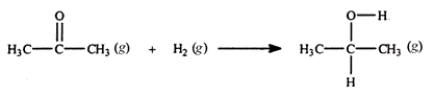
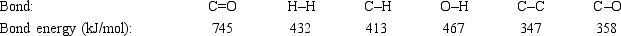
A) −484 kJ
B) −366 kJ
C) −61 kJ
D) +61 kJ
E) +366 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Select the most polar bond amongst the
Q7: Which one of the following properties is
Q8: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF
Q9: Select the compound with the highest lattice
Q10: In which of these substances are the
Q12: Which of the following compounds displays the
Q13: The lattice energy of CaF<sub>2</sub> is the
Q14: Which one of the following properties is
Q15: When one mole of each of the
Q16: Which of the following is a covalent