Multiple Choice
Select the classification for the following reaction. 2NaCl(l) 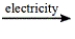 2Na(l) + Cl2(g)
2Na(l) + Cl2(g)
A) Acid-base
B) Precipitation
C) Combination
D) Displacement
E) Decomposition
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q101: Identify the oxidizing agent in the following
Q102: Calcium chloride is used to melt ice
Q103: 1.0M aqueous solutions of the following substances
Q104: Aqueous potassium iodate (KIO<sub>3</sub>) and potassium iodide
Q105: During a titration, the following data were
Q106: How many moles of H<sup>+</sup>(aq) ions are
Q107: All acid-base reactions produce a salt and
Q108: Sodium thiosulfate, Na<sub>2</sub>S<sub>2</sub>O<sub>3</sub>, is used as a
Q109: Consider the reaction: 3Co<sup>2+</sup>(aq) + 6NO<sub>3</sub>(aq) +
Q111: The oxidation numbers of P, S, and