Multiple Choice
Select the classification for the following reaction. CaCl2·H2O(s) 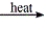 CaCl2(s) + H2O(g)
CaCl2(s) + H2O(g)
A) Combination
B) Decomposition
C) Displacement
D) Acid-base
E) Precipitation
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q44: Copper(II) sulfide, CuS, is used in the
Q45: What volume of a 0.100 M solution
Q46: Select the precipitate that forms when the
Q47: What volume, in L, of 10.0 M
Q48: Magnesium carbonate, MgCO<sub>3</sub>, is insoluble. Identify the
Q50: Which of the following is a strong
Q51: A 0.150 M sodium chloride solution is
Q52: Predict the product(s) for the following reaction.
Q53: Which one of the following substances, when
Q54: In an acid-base (neutralization) reaction the indicator