Multiple Choice
The isotopes of promethium, 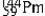 and
and 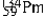 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
A) Promethium-144, β decay; Promethium-134, positron decay
B) Promethium-144, positron decay; Promethium-134, β decay
C) Promethium-144, positron decay; Promethium-134, electron capture
D) Promethium-144, electron capture; Promethium-134, positron decay
E) Promethium-144, β decay; Promethium-134, γ decay
Correct Answer:

Verified
Correct Answer:
Verified
Q59: The radiochemist, Will I. Glow, studied thorium-232
Q60: Which of the following series of radioactive
Q61: The isotopes <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotopes
Q62: Detection of radiation by a Geiger-Müller counter
Q63: After 4 half-lives, the fraction of a
Q65: An isotope with a high value of
Q66: The r-process occurs during supernova explosions.
Q67: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt=" In the equation
Q68: Select the nuclide that completes the following
Q69: Sodium-21 will emit positrons each having an