Multiple Choice
The isotope 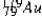 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 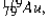 approximately how many such atoms were there present 30 seconds earlier?
approximately how many such atoms were there present 30 seconds earlier?
A) 576
B) 1152
C) 2304
D) 4320
E) 4.30 × 10 8
Correct Answer:

Verified
Correct Answer:
Verified
Q50: In living organisms, C-14 atoms disintegrate at
Q51: The radioisotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The radioisotope
Q52: Iodine-131, t<sub>1/2</sub> = 8.0 days, is used
Q53: So-called "magic numbers" of particles are thought
Q54: Which of the following cannot be an
Q56: A scintillation counter<br>A)measures the signal coming from
Q57: The isotope <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8248/.jpg" alt="The isotope
Q58: Radioactive decay follows zero-order kinetics.
Q59: The radiochemist, Will I. Glow, studied thorium-232
Q60: Which of the following series of radioactive