Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 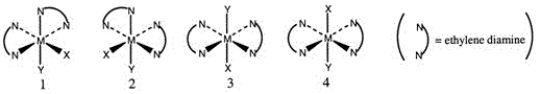 Which one, if any, of the following is a pair of optical isomers?
Which one, if any, of the following is a pair of optical isomers?
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 3 and 4
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Chromium and manganese are among the transition
Q19: Iron(III) forms an octahedral complex with the
Q20: A certain transition element has the stable
Q21: Which of the following ions could exist
Q22: 10.0 mL of a 0.100 mol/L solution
Q24: The maximum oxidation state of an element
Q25: Which of the following complexes could have
Q26: A certain transition metal complex has the
Q27: The ground state electron configuration of a
Q28: Which of the following ligands could participate