Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 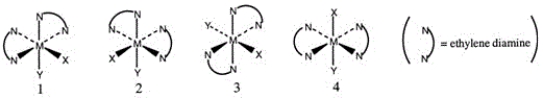 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
A) Structures 1 and 2 are optical isomers.
B) Structures 1 and 3 are optical isomers.
C) Structures 1 and 3 are different complexes.
D) Structures 1 and 4 are geometrical isomers.
E) Structures 3 and 4 are the same complex.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: The oxidation and coordination numbers of cobalt
Q2: Which of the following species could exist
Q3: Which of the following will be the
Q4: Which of the following statements about lanthanides
Q6: The conversion of the chromate ion (CrO<sub>4</sub><sup>2</sup><sup>−</sup>)
Q7: The inner transition series of elements arise
Q8: Of the 3d transition series of elements,
Q9: If a solution absorbs green light, what
Q10: The permanganate ion (MnO<sub>4</sub><sup>−</sup>) is a powerful
Q11: The crystal field splitting energy, Δ,<br>A)is larger