Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 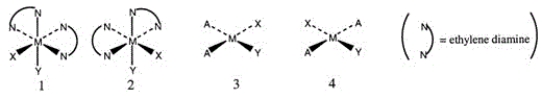 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) 1 and 2 are superimposable.
B) 1 and 2 are geometric isomers.
C) 3 and 4 are structural isomers.
D) 3 and 4 are optical isomers.
E) 3 and 4 are geometric isomers.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: In the spectrochemical series, which one of
Q41: What is the highest possible oxidation state
Q42: According to valence bond theory, what would
Q43: The M<sup>2+ </sup>ions of the first transition
Q44: If M represents a transition element, which
Q46: When the ethylenediaminetetraacetate ion (EDTA<sup>4</sup><sup>-</sup>) forms a
Q47: How many unpaired electrons are there in
Q48: Write the formula for pentaamminechlorocobalt(III) chloride.<br>A)[Co(NH <sub>3</sub>)<sub>5</sub>Cl]Cl<br>B)[Co(NH
Q49: In the compound [Ni(en)<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub>]SO<sub>4</sub> (where en =
Q50: The ground state electronic configuration of Zn<sup>2+</sup>