Multiple Choice
Consider the figure that shows ΔG° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that 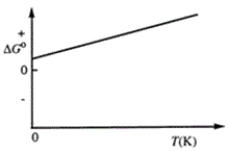
A) Δ H° > 0, Δ S° > 0
B) Δ H° > 0, Δ S° < 0
C) Δ H° < 0, Δ S° > 0
D) Δ H° < 0, Δ S° < 0
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the free energy change, ΔG°,
Q11: You are given pure samples of ammonia,
Q12: As a chemical reaction proceeds toward equilibrium,
Q13: Elemental boron can be formed by reaction
Q14: A sample of water is heated at
Q16: Sulfuryl dichloride is formed when sulfur dioxide
Q17: Consider the following quantities used in thermodynamics:
Q18: In which one of these pairs will
Q19: Which of the following is true for
Q20: Which of the following values is based