Multiple Choice
A reaction has ΔG = 10.0 kJ and ΔG° = 15.0 kJ at a temperature of 50°C. Calculate the value of the reaction quotient 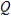 under these conditions.
under these conditions.
A) 0.16
B) 9.1 × 10 −5
C) 1.1 × 10 4
D) 6.4
E) 6.0 × 10 −6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: For a chemical reaction to be spontaneous
Q2: The term microstate refers to the energy
Q3: "A diamond is forever" is one of
Q4: Which one of the following phase changes
Q6: Hydrogen sulfide decomposes according to the following
Q7: Calculate the equilibrium constant at 25°C for
Q8: Which of the following should have the
Q9: Given: H<sub>2</sub>O(l) → H<sub>2</sub>O(s) ΔH° = −6.02
Q10: What is the free energy change, ΔG°,
Q11: You are given pure samples of ammonia,