Multiple Choice
Which one of the following is the best representation of the titration curve that will be obtained in the titration of a weak diprotic acid H2A (0.10 mol L−1) with a strong base of the same concentration? 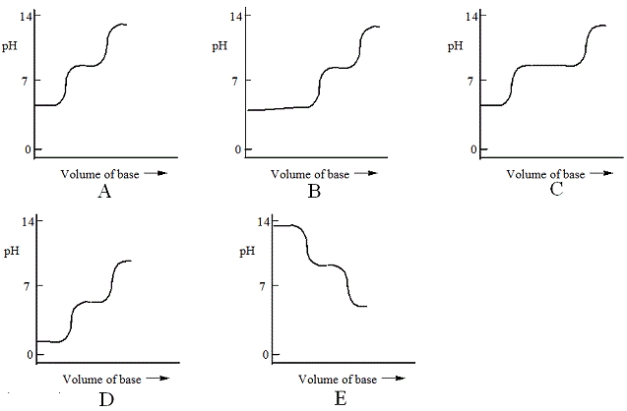
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Q104: You need to prepare a buffer solution
Q105: A 50.0-mL sample of 0.50 M HCl
Q106: When a weak acid is titrated with
Q107: A sample of a monoprotic acid (HA)
Q108: What is the maximum amount of sodium
Q109: The salts X(NO<sub>3</sub>)<sub>2 </sub>and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q110: Write the ion product expression for magnesium
Q111: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q113: The salts X(NO<sub>3</sub>)<sub>2 </sub>and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q114: A popular buffer solution consists of carbonate