Multiple Choice
Write the ion product expression for silver sulfide, Ag2S.
A) 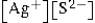
B) 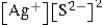
C) 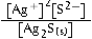
D) 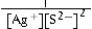
E) 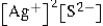
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: A 25.0-mL sample of 0.10 M C<sub>2</sub>H<sub>3</sub>NH<sub>2
Q42: Which of the following has the highest
Q43: The salts X(NO<sub>3</sub>)<sub>2 </sub>and Y(NO<sub>3</sub>)<sub>2</sub> (where X<sup>+</sup>
Q44: A phosphate buffer (H<sub>2</sub>PO<sub>4</sub><sup>−</sup>/HPO<sub>4</sub><sup>2</sup><sup>−</sup>) has a pH
Q45: A 20.0-mL sample of 0.30 M HBr
Q47: A 20.0-mL sample of 0.50 M H<sub>2</sub>C<sub>6</sub>H<sub>6</sub>O<sub>6
Q48: When a weak acid is titrated with
Q49: What is the pH of a solution
Q50: If 10.0 g of NaF and 20.0
Q51: For a diprotic acid H<sub>2</sub>A, the relationship