Multiple Choice
Write the ion product expression for calcium phosphate, Ca3(PO4) 2.
A) 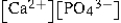
B) 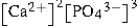
C) 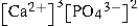
D) 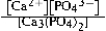
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: Which one of the following is the
Q62: A 20.0-mL sample of 0.30 M HClO
Q63: Calculate the solubility of silver oxalate, Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,
Q64: When 20.0 mL of 0.15 M hydrochloric
Q65: Calculate the solubility of silver phosphate, Ag<sub>3</sub>PO<sub>4</sub>,
Q67: Calculate the solubility of magnesium sulfate, MgSO<sub>4</sub>,
Q68: At the equivalence point in an acid-base
Q69: Buffer solutions with the component concentrations shown
Q70: Citric acid has an acid dissociation constant
Q71: Which of the following acids should be