Multiple Choice
Write the mass-action expression, Qc, for the following chemical reaction equation. 2C6H6(g) + 15O2(g) ⇄ 12CO2(g) + 6H2O(g)
A) 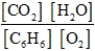
B) 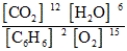
C) 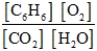
D) 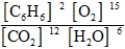
E) 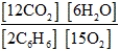
Correct Answer:

Verified
Correct Answer:
Verified
Q49: In water, the following equilibrium exists: H<sup>+</sup>(aq)
Q50: Consider the following two equilibria and their
Q51: Consider the equilibrium reaction shown below. B<sub>2</sub>(g)
Q52: Consider the reversible reaction: 2NO<sub>2</sub>(g) ⇄ N<sub>2</sub>O<sub>4</sub>(g)
Q53: When a reaction system reaches equilibrium, the
Q55: Unless ΔH°<sub>rxn</sub> = 0, a change in
Q56: The equilibrium constant for the reaction of
Q57: Consider the equilibrium reaction: H<sub>2</sub>(g) + Br<sub>2</sub>(g)
Q58: At 850°C, the equilibrium constant K<sub>p</sub> for
Q59: Carbon monoxide and chlorine combine in an