Multiple Choice
Write the mass-action expression, Qc, for the following chemical reaction. 3ClO2−(aq) ⇄ 2ClO3−(aq) + Cl−(aq)
A) 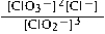
B) 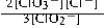
C) 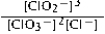
D) 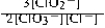
E) 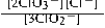
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: The equilibrium constant, K<sub>p</sub>, has a value
Q9: Increasing the initial amount of the limiting
Q10: Hydrogen iodide, HI, is formed in an
Q11: If all of the coefficients in the
Q12: Methanol can be synthesized by combining carbon
Q14: The two equilibrium constants for the same
Q15: The reaction of nitric oxide to form
Q16: For a gas-phase equilibrium, a change in
Q17: Nitric oxide is formed in automobile exhaust
Q18: The reaction system CS<sub>2</sub>(g) + 4H<sub>2</sub>(g) ⇄