Multiple Choice
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 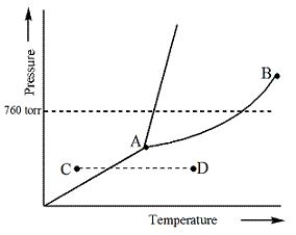
A) Increasing temperature with a phase change from solid to liquid
B) Increasing temperature with a phase change from solid to vapor
C) Increasing temperature with a phase change from liquid to vapor
D) Increasing temperature with no phase change
E) Increasing temperature beyond the critical point
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which of the following statements about the
Q7: Which one of the following quantities is
Q8: The highest temperature at which superconductivity has
Q9: The phase diagram of a substance can
Q10: Which of the following liquids is likely
Q12: The coordination number of sodium and chloride
Q13: Which of the following should have the
Q14: Which of the following pairs is arranged
Q15: Crystal structures may be conveniently measured using<br>A)X-ray
Q16: In a transistor, the current through one