Multiple Choice
In the following Lewis structure for phosphate, phosphorus has a formal charge of __________ and an oxidation number of __________. 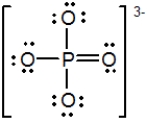
A) 0; −3
B) 0; 5
C) 5; −3
D) 5; 5
E) 3; 5
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: Select the correct Lewis structure for NOCl,
Q15: In which one of the following species
Q16: Which one of the following molecules does
Q17: The molecule AX<sub>2</sub>, where A and X
Q18: How many resonance structures are possible for
Q20: In order for a noncyclic triatomic molecule
Q21: Use VSEPR theory to decide which one
Q22: What is the molecular shape of BCl<sub>3
Q23: Use VSEPR theory to decide which one
Q24: According to VSEPR theory, a molecule with