Multiple Choice
In which of the following does the nitrogen atom have a formal charge of −1?
A) 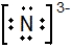
B) 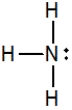
C) 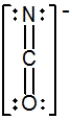
D) 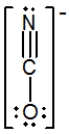
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: What is the molecular shape of BCl<sub>3
Q23: Use VSEPR theory to decide which one
Q24: According to VSEPR theory, a molecule with
Q25: How many electron pairs are shared between
Q26: Predict the actual bond angles in SF<sub>3</sub><sup>+</sup>
Q28: What is the molecular shape of NO<sub>2</sub><sup>−</sup>
Q29: Which one of the following Lewis structures
Q30: What is the molecular shape of BeH<sub>2
Q31: According to VSEPR theory, a molecule with
Q32: What is the molecular shape of ClCN