Multiple Choice
The formal charges on Cl and O in the structure shown for the ClO− ion are, respectively 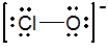
A) 0 and −1
B) −1 and 0
C) 1 and −2
D) −2 and 1
E) None of these choices are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q59: Use VSEPR theory to predict the electron
Q60: Predict the ideal bond angles in FNO
Q61: In which one of the following species
Q62: According to VSEPR theory, a molecule with
Q63: List all possible molecular geometries (shapes) for
Q65: What is the molecular shape of HOF
Q66: What is the molecular shape of NOCl
Q67: According to VSEPR theory, a molecule with
Q68: In the nitrate ion (NO<sub>3</sub><sup>−</sup>), nitrogen and
Q69: What is the molecular symmetry around the