Multiple Choice
In which one of the following structures does the central atom have a formal charge of +2?
A) 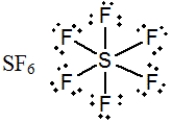
B) 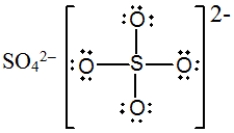
C) 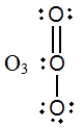
D) 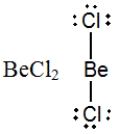
E) 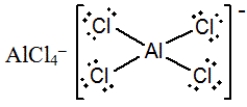
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: Which one of the following Lewis structures
Q75: Which of the following elements would you
Q76: Considering all the bonds in a molecule
Q77: Oxygen difluoride is a powerful oxidizing and
Q78: What is the molecular shape of ClO<sub>3</sub>F
Q80: According to VSEPR theory, a molecule with
Q81: Select the correct Lewis structure for nitrogen
Q82: According to VSEPR theory, a molecule with
Q83: Thionyl chloride is used as an oxidizing
Q84: In which one of the following species