Multiple Choice
What is the molecular shape of N2O as predicted by the VSEPR theory? 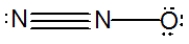
A) Trigonal pyramidal
B) Trigonal planar
C) Angular
D) Bent
E) Linear
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q88: Which of the following has no net
Q89: The best Lewis structure for sulfuric acid
Q90: In carbon disulfide, how many lone pairs
Q91: Predict the actual bond angle in SeCl<sub>2
Q92: According to VSEPR theory, a molecule with
Q93: Select the Lewis structure in which formal
Q95: Which one of the following Lewis structures
Q96: According to VSEPR theory, a molecule with
Q97: Predict the smallest actual bond angle in
Q98: According to VSEPR theory, a molecule with