Multiple Choice
The figure shows a molecular-level representation of the following voltaic cell: Mg(s) + Sn2+(aq) → Mg2+(aq) + Sn(s) When drawing the "after" representation one would note that 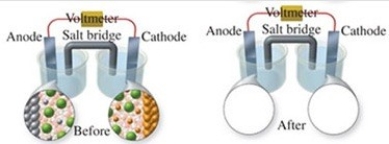
A) the tin electrode will be smaller.
B) the magnesium electrode will be larger.
C) the number of Mg2+ ions in solution will remain constant.
D) deposits will form in the salt bridge.
E) the number of Sn2+ ions in solution will decrease.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Given the following information about the activity
Q2: The potential of a voltaic cell is
Q3: Equations that are written to represent either
Q4: Consider the following oxidation-reduction reaction: 2Fe<sup>3+</sup>(aq)+ 2Hg(l)+
Q5: Given the following information about the activity
Q7: The figure shows the electrolysis of molten
Q8: Consider the reaction: N<sub>2</sub>(g)+ 3H<sub>2</sub>(g)→ 2NH<sub>3</sub>(g)Which of
Q9: Consider a voltaic cell that corresponds to
Q10: In which compound does phosphorus have an
Q11: An electrolytic cell is an electrochemical cell