Multiple Choice
The figure shows the electrolysis of molten NaI. What reaction occurs at the cathode of this cell? 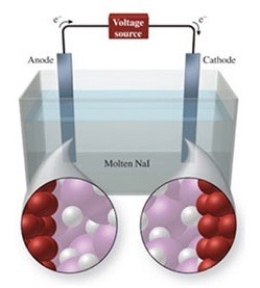
A) NaI(l) → NaI2(l)
B) I−(l) + e− → I2−
C) Na+(l) + e− → Na(l)
D) 2I−(l) + e− → I2(g)
E) 2I−(l) → I2(g) + 2e−
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Given the following information about the activity
Q14: The following reaction occurs in a lead
Q15: Consider the reaction: Ca(s)+ 2H<sub>2</sub>O(l)→ Ca(OH)<sub>2</sub>(aq)+ H<sub>2</sub>(g)Which
Q16: The change, Br<sub>2</sub> + H<sub>2</sub>O → HOBr
Q17: Consider the reaction: Zn(s)+ H<sub>2</sub>SO<sub>4</sub>(aq)→ ZnSO<sub>4</sub>(aq)+ H<sub>2</sub>(g)Which
Q19: In a fuel cell that uses gaseous
Q20: In any electrolytic cell, the cathode is
Q21: Consider the half-reaction NO<sup>3</sup>−(aq)→ NO(aq). When the
Q22: Consider the reaction: H<sub>3</sub>AsO<sub>3</sub>(aq)+ BiO<sub>3</sub><sup>−</sup>(aq)→ H<sub>3</sub>AsO<sub>4</sub>(aq)+ Bi(s)When
Q23: Consider a voltaic cell that corresponds to