Multiple Choice
The figure shows the electrolysis of molten AlCl3. What reaction occurs at the cathode of this cell? 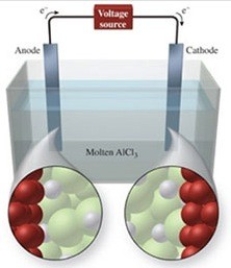
A) AlCl3(l) → AlCl2(l)
B) Cl-(l) + e- → Cl2-
C) Al3+(l) + 3e- → Al(l)
D) 2Cl-(l) + 2e- → Cl2(g)
E) 2Cl-(l) → Cl2(g) + 2e-
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Consider a voltaic cell that corresponds to
Q24: The balanced half-reaction for the oxidation of
Q25: The products of the electrolysis of molten
Q26: Consider the reaction: CH<sub>4</sub>(g)+ 2O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+ 2H<sub>2</sub>O(g)Which
Q27: Consider the reaction: Sn<sup>2+</sup>(aq)+ 2Fe<sup>3+</sup>(aq)→ Sn<sup>4+</sup>(aq)+ 2Fe<sup>2+</sup>(aq)Which
Q29: Consider the reaction: H<sub>2</sub>O(l)+ 3SO<sub>3</sub><sup>2−</sup>(aq)+ 2MnO<sub>4</sub><sup>−</sup>(aq)→ 3SO<sub>4</sub><sup>2−</sup>(aq)+
Q30: Consider the reaction: Zn(s)+ H<sub>2</sub>SO<sub>4</sub>(aq)→ ZnSO<sub>4</sub>(aq)+ H<sub>2</sub>(g)Which
Q31: Consider a voltaic cell that corresponds to
Q32: Consider the reaction: Zn(s)+ NO<sub>3</sub><sup>−</sup>(aq)→ NH<sub>3</sub>(aq)+ Zn(OH)<sub>4</sub><sup>2−</sup>(aq)When
Q33: Reduction is a chemical process in which