Multiple Choice
The figure shows the electrolysis of molten AlCl3. What is the balanced equation for the reaction? 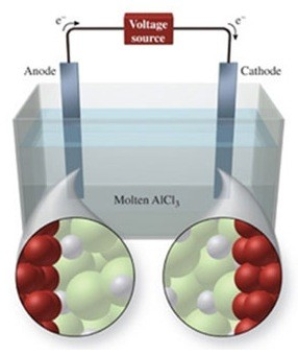
A) AlCl3(l) → Al3+(l) + 3Cl−(l)
B) AlCl3(l) → Al(l) + Cl3(g)
C) 2AlCl3(l) → 2Al(l) + 3Cl2(g)
D) AlCl3(s) → Al3+(aq) + 3Cl−(aq)
E) 2AlCl3(s) → 2Al(s) + 3Cl2(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q49: The reaction that occurs in an alkaline
Q50: The chemical reactions that occur in voltaic
Q51: A voltaic cell is prepared in which
Q52: Examine the following reaction: 5FeCl<sub>2</sub>(aq)+ KMnO<sub>4</sub>(aq)+ 8HCl(aq)→
Q53: In which of the following choices is
Q55: What is the oxidation number of chlorine
Q56: What are the oxidation numbers of the
Q57: List the oxidation number of sulfur in
Q58: In which of the following choices is
Q59: Given the following reaction in a voltaic