Multiple Choice
The image shows a molecular-level representation of part of a solution after ammonia, NH3, is dissolved in water. (Besides water, there are 2 NH4+ ions, 6 NH3 molecules, and 2 OH− ions present in the solution.) . Which of the following best describes NH3? 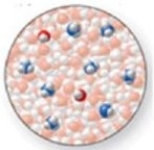
A) strong acid
B) strong base
C) weak acid
D) weak base
E) amphoteric
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Identify orange juice, pH = 3.5, as
Q10: Given an H<sub>3</sub>O<sup>+</sup> concentration of 1.0 ×
Q11: Which of the following ionizes to the
Q12: Which of the following statements regarding bases
Q13: Which of the following ionizes to the
Q15: How does a buffered solution resist changes
Q16: All of the following species are weak
Q17: If you take an antacid tablet, the
Q18: When fluoride ion, F<sup>−</sup>, reacts with water,
Q19: Is it possible for an indicator to