Multiple Choice
Three phases of water are shown in the figure. List the terms used to identify the phase changes indicated by the arrows. 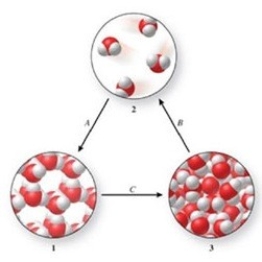
A) A = sublimation, B = vaporization, C = freezing
B) A = condensation, B = sublimation, C = melting
C) A = freezing, B = vaporization, C = melting
D) A = deposition, B = vaporization, C = melting
E) A = deposition, B = sublimation, C = freezing
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Which substance has the highest melting point?<br>A)Cu<br>B)C<sub>6</sub>H<sub>14</sub><br>C)Rn<br>D)C
Q25: The diagram represents the physical state of
Q26: Rank the following substances in order of
Q27: Which of the following statements regarding intermolecular
Q28: Rank the following substances in order of
Q30: Which of the following statements regarding the
Q31: CH<sub>3</sub>OH boils at 64.7°C, and C<sub>2</sub>H<sub>6</sub> boils
Q32: Which of the following substances is most
Q33: What phase change is occurring in the
Q34: One would expect that since CO is