Multiple Choice
What phase transition is occurring between points B and C on the heating curve? 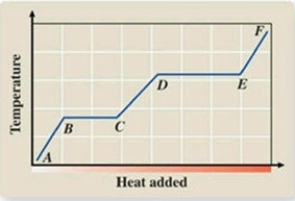
A) melting
B) condensation
C) evaporation
D) sublimation
E) deposition
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: Identify the type of solid shown in
Q78: Calculate the amount of heat energy required
Q79: A solid substance has a very high
Q80: From the vapor pressure curve of acetone,
Q81: Identify the type of solid shown in
Q83: Network covalent substances have the highest melting
Q84: Which of the following statements regarding the
Q85: Rank the following substances in order of
Q86: Consider the melting point of the following
Q87: Which of the following substances can participate