Multiple Choice
What phase transition is occurring between points D and E on the heating curve? 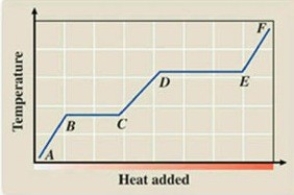
A) melting
B) freezing
C) evaporation
D) sublimation
E) deposition
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q97: The forces that hold CO<sub>2</sub> together in
Q98: Which of the following molecules experience dipole-dipole
Q99: Calculate the amount of heat energy required
Q100: Which of the following substances can participate
Q101: Which of the following statements is correct?<br>A)A
Q103: If there were no intermolecular forces, most
Q104: Which of the following has the highest
Q105: Which of the molecules in the figure
Q106: A solid substance has a very low
Q107: What causes water to have a high